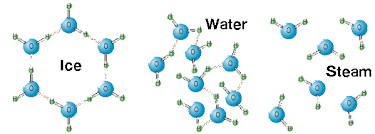
Phase transition from liquid to solid (i.e., solidification) involves intermolecular forces of attraction that lowers the energy of motion. The reverse process of melting is where a solid transitions to a liquid . Liquid water freezes at 0° and colder., and ice starts to melt at 0°, and warmer. This temperature is
As water gets colder to freeze, or warmer to melt, the water changes temperature as it absorbs energy (heating) or loses energy (cooling).
But at the temperature of the phase change, the energy of the system is only used to change phase. The temperature does not change. At this temperature (0°) adding thermal energy to ice will disrupt the attractive intermolecular forces, causing it to melt.
If the water were liquid and at 0°, removing thermal energy (cooling the water) reduces the kinetic energy (the energy of motion) which allows the intermolecular forces to pull the water molecules closer together, which further limits their motion and allows the molecules to organize in a more regular pattern (i.e., decreasing the entropy of the water). The water still does not change temperature as it goes from a liquid to a solid.
It's kindasorta an energetic version of a car spinning it's wheels in the ditch. The wheels in this analogy is the kinetic energy of the system being used to change phase. It’s only until the wheels stop spinning (phase change complete) that the car can go forward or backward (that the temperature can increase or decrease).
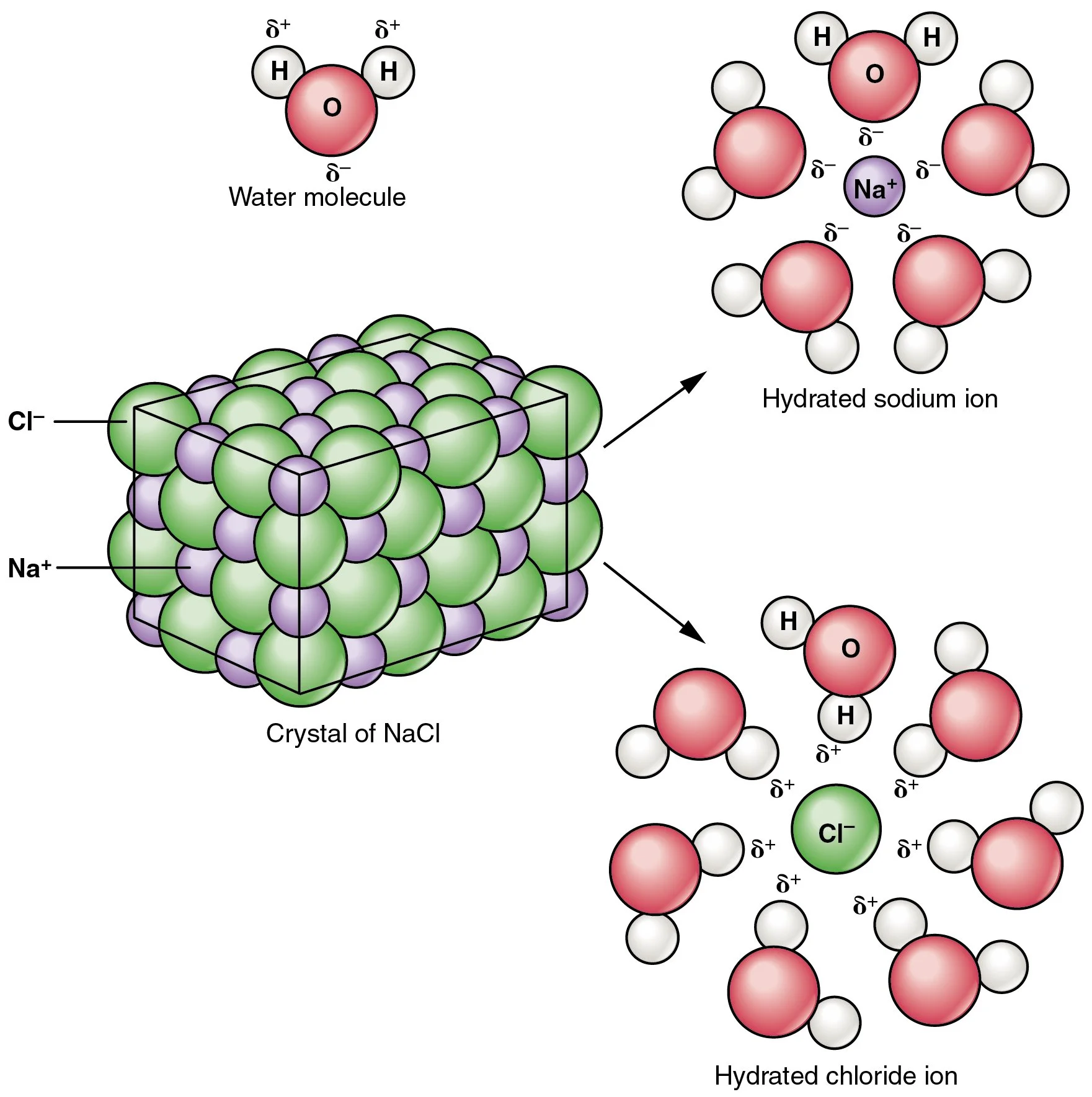
Adding particulates can disrupt that bonding because they literally get in the way of the water molecules, obstructing the attraction of the intermolecular forces. It takes even more energy to be removed from the system for the intermolecular forces to properly attract. Any particulate that is small enough will do this. In the case of salt, putting it into a solution with water causes the ions to dissociate, making even smaller particles. The bottom example is of NaCl, but any salt will do this. Salts such as Magnesium Chloride will dissociate into 2 atoms of Mg, so that gives 3 particles per molecule in comparison to the 2 particles of NaCl.
The process of adding a particulate to lower the freezing point is called freezing point depression and it is considered a colligative property: a property that depends on the number of particles used.
resources, a.k.a. fair use fair use
[1] https://sciencenotes.org/why-salt-makes-ice-colder-how-cold-ice-gets/
[2] https://www.khanacademy.org/science/ap-chemistry-beta/x2eef969c74e0d802:chemical-reactions/x2eef969c74e0d802:net-ionic-equations/a/complete-ionic-and-net-ionic-equations